HeartSine Defibrillator Product Recall – June 2025

HeartSine Defibrillator Product Recall – June 2025
Stryker Australia has issued an urgent product recall for select HeartSine Samaritan PAD Automated External Defibrillators (AEDs) following the identification of a potential manufacturing issue. This recall is being conducted as a precaution to ensure continued public safety
Product Description
The HeartSine Samaritan PAD is a lightweight, battery-operated AED designed to deliver a life-saving shock in cases of sudden cardiac arrest (SCA). It is commonly used in public, workplace, and community settings.
Who Is Affected?
This recall applies to a subset of the following HeartSine Samaritan PAD models:
- 350P
- 360P
- 500P
Affected serial numbers:
- Begin with 21, 22, 23, or 24
- Followed by the letter B, D, E, G, or H
Check your HeartSine Defibrillator here: Stryker recall website
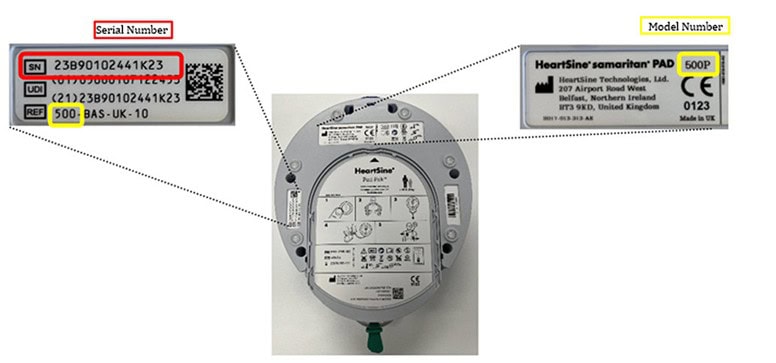
Locate the serial number on the rear label of your AED. You can enter it into the search tool on the Stryker recall website to confirm if your device is affected.
What’s the Issue?
During internal quality testing, a manufacturing issue related to a circuit board component was identified.
- This issue may impair the device’s ability to function or deliver therapy.
- No failures have been reported during actual patient use.
- Risk is extremely low — most devices are expected to perform as intended.
Next Steps for Customers
If your device is affected:
- Stryker will contact you directly to confirm the recall and arrange for a replacement AED.
- Replacements will be provided, subject to availability. More details on timing will follow.
- Continue using your current AED unless otherwise advised. Internal testing confirms a low likelihood of malfunction.
Device Requirements During Recall
- Keep AEDs in service until a replacement is provided.
- Document the model and serial number of all devices (impacted and non-impacted).
Stay Informed
Updates and further instructions will be shared by Stryker directly. In the meantime, if you have any concerns or require assistance, please contact Stryker on this email [email protected] or refer to the contact details in your official recall letter.
Your safety is our priority.
Thank you for your cooperation as we work together to ensure the continued reliability of life-saving AEDs in your community.
 Construction Defibrillator Bundles
Construction Defibrillator Bundles